Treat Endopain
Latest Conference Coverage

Kevin Ault, MD, on vaccines & autism, local government, HPV vaccine advocacy

Study data highlight trade-offs of testing for pregnancy too soon

Barbara Levy, MD, on importance of public comments on new cervical cancer screening guidance
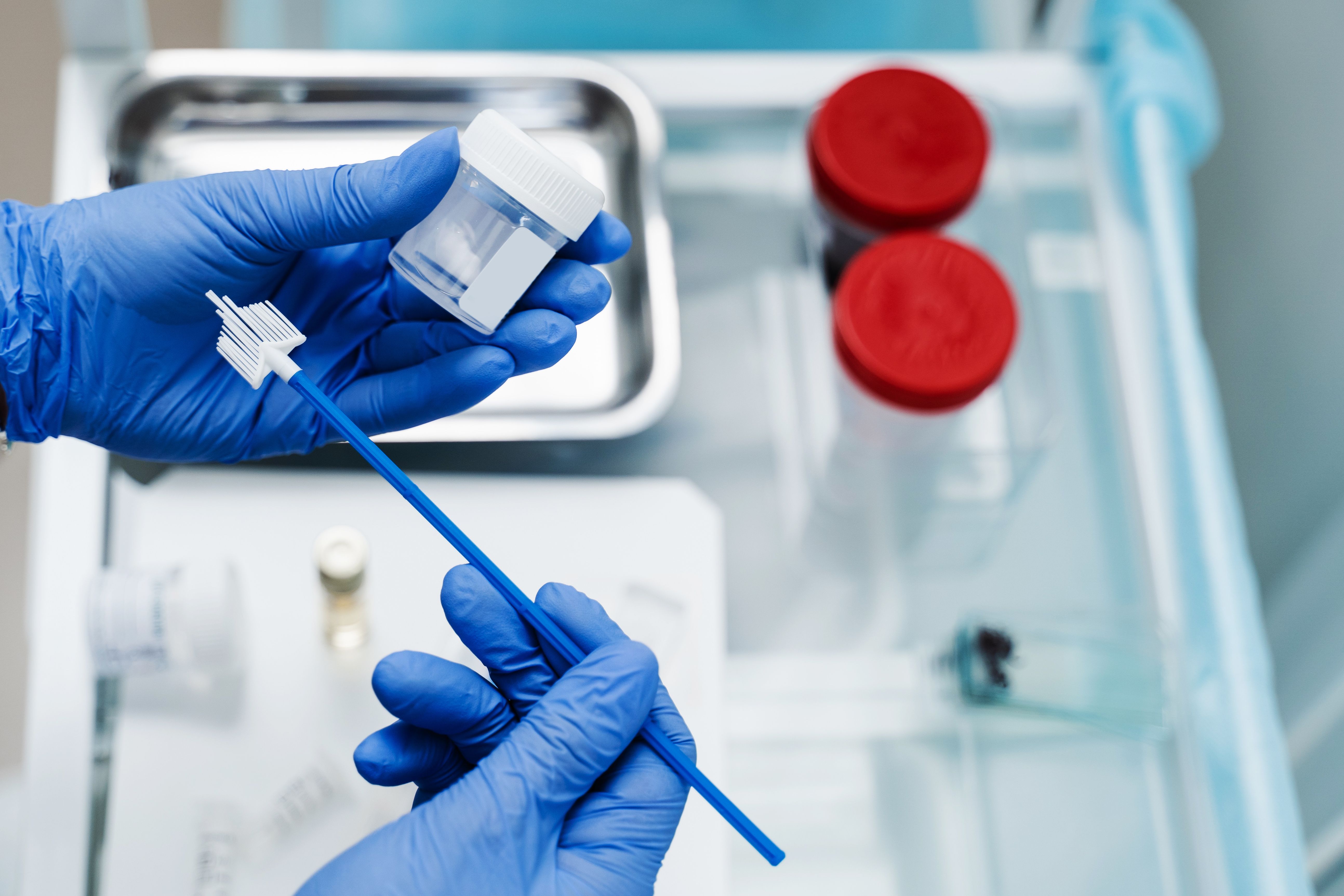
Minipad-based HPV testing shows promise for cervical cancer screening

Kevin Ault, MD, on maternal immunization, following the correct recommendations

ACOG calls for safe, equitable ob-gyn care for immigrants

FDA approves Aptima HPV assay for clinician-collected primary cervical cancer screening

Pregnancy vaccination FAQs: ACOG guidance on COVID-19, flu, and RSV

Contemporary OB/GYN's top video interviews from January 2026

Recapping January's top Contemporary OB/GYN headlines
