
A recent investigation finds comparable safety and effectiveness between intravenous and intramuscular oxytocin administration, suggesting intramuscular administration may be preferable for postpartum hemorrhage prevention.

A recent investigation finds comparable safety and effectiveness between intravenous and intramuscular oxytocin administration, suggesting intramuscular administration may be preferable for postpartum hemorrhage prevention.

A recent investigation sheds light on patient preferences and concerns regarding external cephalic version versus cesarean section for managing fetal malpresentation, revealing gaps in counseling and the desire for more information.

Recent research highlighted the significant correlation between adverse childhood experiences and heightened risks of preeclampsia and depression during pregnancy.

Following a landmark decision regarding the status of embryos, the University of Alabama at Birmingham has suspended in vitro fertilization services, sparking concerns over reproductive rights and health care access as experts grapple with the legal aftermath.
Recent research highlighted an association between the total dose of prescribed opioids during pregnancy and the heightened risk of spontaneous preterm birth, emphasizing the need for judicious opioid use in pain management for expectant mothers.

Discover key findings from a comprehensive study on in vitro fertilization outcomes among women with obesity, shedding light on the importance of equitable access to fertility treatment regardless of body mass index.

Review some of the top stories from the Contemporary OB/GYN website over the last week, and catch up on anything you may have missed.

New research from ISC 2024 reveals an increase in stroke risk for Black women with treatment-resistant hypertension before age 35, underscoring critical disparities in healthcare.

A new study found that opioid morphine milligram equivalents dose prescribed in the 60 days prior to delivery date was significantly associated with a higher likelihood of spontaneous preterm birth.

Recent research delves into the repercussions of gestational diabetes mellitus on both maternal and perinatal health, shedding light on its effects in both singleton and twin pregnancies.

Delve into the intricate correlation between human chorionic gonadotropin levels during pregnancy and various adverse outcomes, shedding light on its multifaceted role in maternal-fetal health.
Adolescent pregnancy and sexual health care utilization declined during the COVID-19 pandemic, indicating significant disruptions in reproductive health services for young females, as highlighted in a recent study published in Pediatrics.

A novel ultrasound technique allows for early and accurate prediction of preterm birth risk, potentially revolutionizing prenatal care for first-time pregnant women.

Delve into the dialogue surrounding women's cardiovascular health with Natalie Bello, MD, MPH, FACC, as she sheds light on the significance of American Heart Month and the impact of pregnancy on cardiovascular risk factors.

Review some of the top stories from the Contemporary OB/GYN website over the last week, and catch up on anything you may have missed.

Samantha Olson, MPH, highlights how maternal vaccination protects infants from influenza.

Nipocalimab, a monoclonal antibody targeting maternal alloantibodies, has received FDA Breakthrough Therapy Designation for treating severe hemolytic disease of the fetus and newborn.

Uncover the impact of maternal pre-pregnancy BMI on perinatal outcomes as elucidated by a multi-study investigation, shedding light on strategies to mitigate obstetric and neonatal risks through weight management.

A study revealed a correlation between maternal cortisol levels, stress, and adverse birth outcomes, shedding light on potential implications for perinatal health.
A recent study highlighted how racial disparities, exacerbated by structural racism, impact the administration of neuraxial analgesia during labor, underscoring the need for interventions to address social inequity in maternal health care access.

Placenta accreta spectrum presents a complex obstetric challenge, characterized by the abnormal adherence of the placenta to the uterine wall, often leading to potentially life-threatening hemorrhage.

Recent research led by David J. Rooney reveals that while folate, vitamin B12, and iron intake show no direct links to fetal parameters, lower maternal hemoglobin concentrations in late pregnancy are associated with faster fetal growth rates, larger birthweights, and increased placental weights.
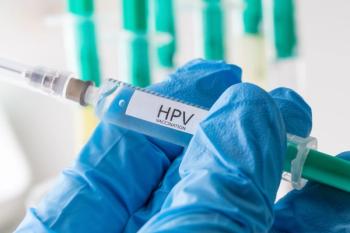
A recent study revealed a 2-dose regimen of human papillomavirus vaccination in postpartum individuals is as effective as a 3-dose approach, providing valuable insights into optimizing vaccination strategies against the virus.

A recent study revealed that early postpartum administration of dexmedetomidine significantly reduces the risk of postpartum depression among women with prenatal depression.

Pregnant women in rural Appalachia face an 11.94% hepatitis C virus infection rate—5 times the state average—revealing a pressing public health concern that demands increased treatment opportunities.