Vulvovaginal disease in the inpatient setting
In part 1 of this review, red flags for severe skin disease are discussed.
Recent data have shown that skin disease was diagnosed in 1 in 8 hospitalized adults, either as the primary reason for admission or as a comorbid condition in patients hospitalized for another reason.1 Thus, performing a full-body skin examination on admitted patients is critical for detecting cutaneous signs of potentially fatal diseases. Female genitalia continues to be an underevaluated area of the body due to both patient and practitioner discomfort. This is particularly problematic, as common skin diseases and cutaneous signs of systemic illness may display different morphologies and nuances when involving the genital area. The problem is compounded by a perception in both gynecology and dermatology of insufficient vulvovaginal training, which can lead to a lack of ownership of this area of the body by either specialty. A recent survey has shown that approximately half of obstetrician-gynecologist residents and program directors, and more than half of dermatology program directors, do not feel training in vulvovaginal disease is adequate.2

This review focuses on the recognition and management of common dermatologic and systemic diseases that may present with vulvovaginal involvement. In part 1, we review red flags for severe skin disease, looking at drug-induced conditions where particular care should be taken to manage vulvovaginal involvement in the inpatient setting. In part 2, we will continue our discussion to cover vulvovaginal involvement in inflammatory and ulcerative conditions in the inpatient setting.
Red flags for severe skin disease
Hospitalized patients can present with a variety of skin lesions. It is important to recognize features upon skin examination and in the patient’s history that may indicate severe skin disease. Concerning morphologies include purpura (especially retiform purpura), blisters, necrosis, and targetoid lesions. Targetoid lesions are characterized by 2 concentric color zones and should be di fferentiated from typical target lesions, which involve 3 zones. Typical target lesions are seen in erythema multiforme, whereas targetoid lesions may be seen in conditions including Stevens-Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN) and fixed drug eruptions. Concerning features include involvement of greater than 50% body surface area (BSA), skin pain, and a positive Nikolsky sign, in which tangential application of pressure to skin leads to shearing of the epidermis from the lower dermis. Concerning systemic signs include fever, myalgias, arthralgias, and edema of the face, hands, or feet. Finally, it is important to note whether the patient has a history of high-risk medication usage, including certain antibiotics, neuroleptics, allopurinol, or nonsteroidal anti-inflammatory drugs (NSAIDs).
Drug-induced conditions
SJS/TEN
SJS/TEN represents a spectrum of severe blistering mucocutaneous skin diseases with high morbidity and mortality. In SJS, patients have skin detachment a ffecting less than 10% of the BSA. In TEN, patients have skin detachment a ffecting greater than 30% of the BSA. In SJS/TEN overlap, patients have skin detachment between 10% and 30% BSA. Individuals of non-White race are disproportionately a ffected.3 Mortality ranges from 4.8% for SJS to 19.4% for SJS/TEN to 14.8% for TEN.3
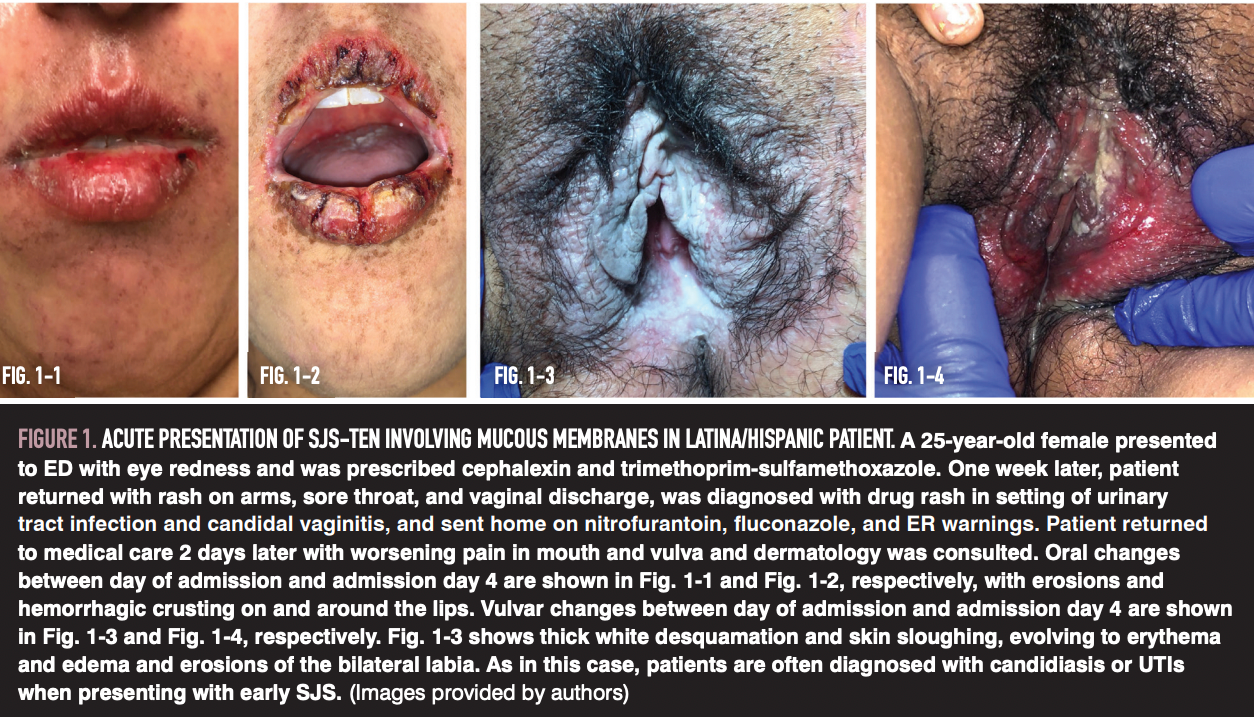
SJS/TEN often involves an identifiable drug trigger. The largest cohort of cases in the United States found that 90% of cases were caused by medication.4 Antibiotics are most commonly implicated (trimethoprim-sulfamethoxazole, -lactams, fluoroquinolones), followed by neuroleptics (phenytoin, lamotrigine, carbamazepine), allopurinol, and NSAIDs.4 Affected patients typically experience fever and an influenzalike prodrome 1 to 3 days before developing mucocutaneous lesions. Other prodromal features include myalgias, arthralgias, fatigue, and early signs of mucosal involvement, including photophobia, dysphagia, and conjunctival itching or burning (Figure 1). Cutaneous lesions typically begin on the face and thorax and include tender to painful, ill-defined coalescing erythematous macules that progress to vesicles and bullae before beginning to slough within days. Close physical examination is important as early changes can be subtle.
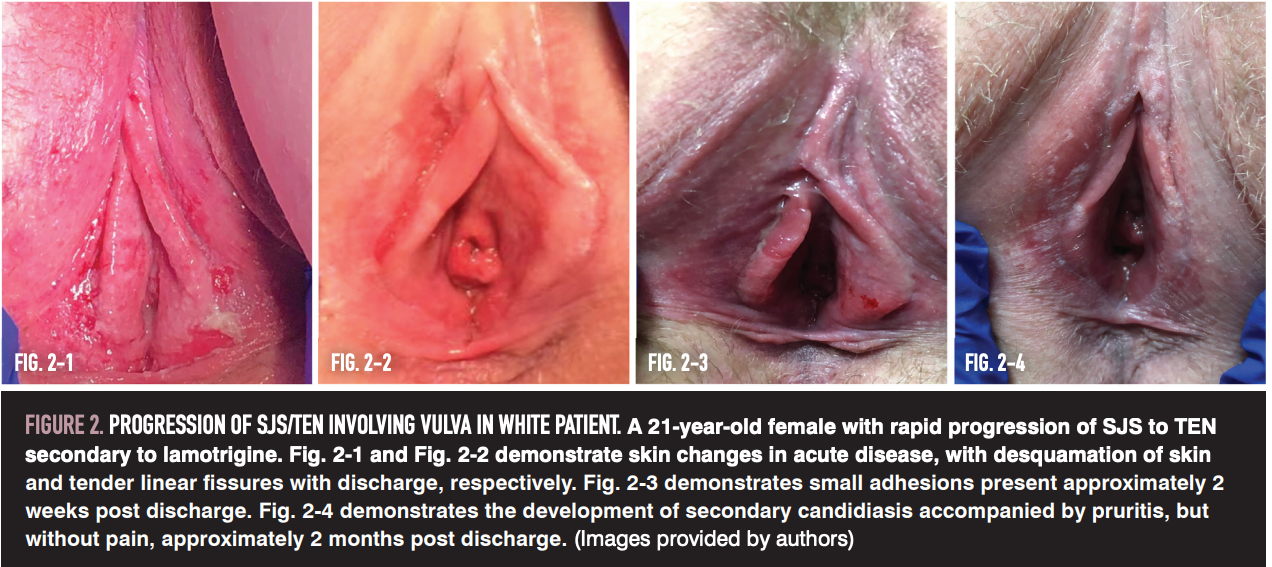
Vulvovaginal involvement affects up to 70% of patients with SJS/TEN and includes widespread vulvovaginal erosions, ulcerations, bullae, and vaginitis.5 Clinicians should strive to recognize vulvovaginal involvement early and treat aggressively to prevent severe complications. There are several possible patterns of progression of vulvovaginal involvement in SJS/TEN. For example, desquamation of skin may lead to discharge and tender linear fissures (Figure 2-1, 2-2). Alternatively, adhesions may form and then separate, leading to secondary candidiasis and pruritus (Figure 2-3, 2-4).
Despite the prevalence of vulvovaginal involvement in SJS/TEN, there is little consensus regarding optimal prevention and treatment strategies, and expert opinion currently guides clinical care. Treatment includes ultrapotent topical and intravaginal steroids, barrier cream, vaginal dilation, and suppression of menstruation until healing of vulvovaginal lesions.6 Urinary catheters should be used to promote urothelial protection and patient comfort.6 Outpatient follow-up 2 weeks after discharge with both gynecology and dermatology is recommended, with continued care every 2 months for the first 6 months. This early and aggressive multidisciplinary management in the months following discharge is essential to preventing irreversible architectural changes.
SJS/TEN survivors often experience chronic physical and psychological sequelae. Chronic physical sequelae include cutaneous changes in 84.3% of patients, ocular sequelae in 59.5% of patients, and oral mucosal problems in 50.8% of patients.7 In addition, up to 28% of patients develop chronic genital sequelae.5 Most commonly, this includes pelvic floor dysfunction and vulvovaginal dryness, pruritus, and burning with concurrent dyspareunia and dysesthesias. In addition, a longitudinal survey has shown that most patients experience depression, and 43% of patients showed signs of anxiety.7 One study has shown that survivors receive inadequate mental health services post acute disease.8 It is important to offer both patients and family members psychological support during hospitalization, before discharge, and through outpatient follow-up. Support groups such as the Stevens-Johnson Syndrome Foundation are another important component of care.
Linear immunoglobulin A disease
Linear immunoglobulin A (IgA) disease is an autoimmune blistering disease characterized by IgA deposition at the dermal-epidermal junction that may be drug induced or idiopathic. Although this disease can occur at any age, adults are typically affected in their teenage years or in the sixth decade of life and frequently have drug-induced disease.9 Vancomycin is the most common culprit, especially in the inpatient setting. Symptoms are generally seen within the first month of drug administration10,11; however, the particular latency period depends on the causative drug. For example, disease secondary to trimethoprim-sulfamethoxazole has been associated with a latency period of 780 days.12
Upon skin examination, adult patients present with scattered tense vesicles or bullae that may arise on a background of nonin flamed skin or on background in ammatory plaques. Lesions are typically widespread and may involve the trunk, extensor extremities, buttocks, and face.13 Mucosal involvement is common, with erosions or ulcers most frequently affecting the oral cavity followed by genitalia.14 Of note, genital involvement is more commonly seen in children, as is the classic “string of pearls” arrangement, in which annular erythematous lesions are accompanied by a ring of vesicles (Figure 3).14,15
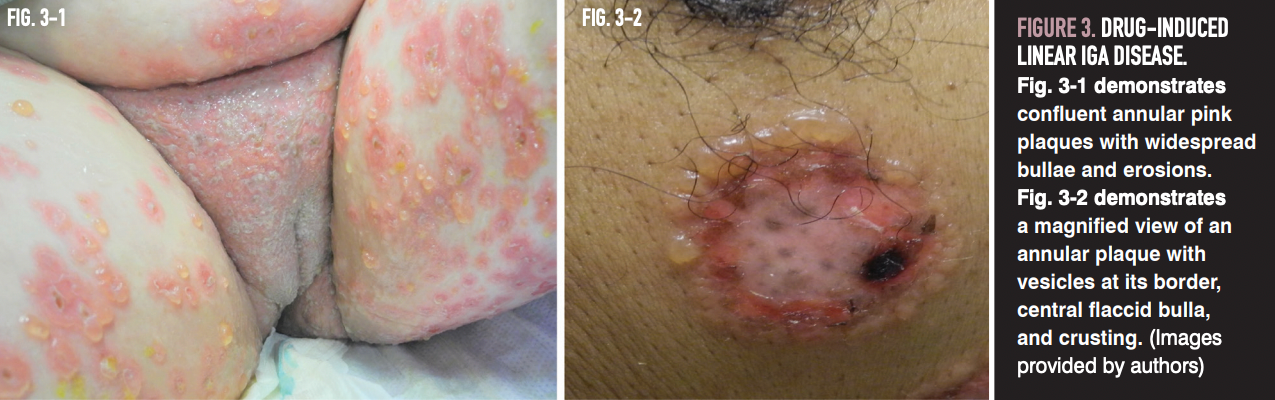
Atypical or heterogeneous presentations may make clinical differentiation from other autoimmune vesiculobullous diseases or TEN difficult. Therefore, biopsy for histopathology is a crucial aspect of working up suspected cases. When drug-induced linear IgA disease is suspected, an algorithm (such as WHO-UMC or Naranjo) to identify the causative drug should be employed.12 Optimal management involves discontinuation of suspicious drugs and systemic therapy if lesions reappear in rechallenge.12 Additional treatment options include low-dose dapsone or, for patients with vancomycin-induced disease, the addition of doxycycline. Complete clinical remission upon suspected drug discontinuation is seen in approximately half of patients, with most cases of drug-induced disease resolving within 2 to 6 weeks of drug discontinuation.12
Acute generalized exanthematous pustulosis
Acute generalized exanthematous pustulosis (AGEP) is a cutaneous eruption characterized by rapid development of multiple nonfollicular sterile pustules on background erythema. Most cases are caused by adverse drug reactions, particularly penicillin or macrolide antibiotics.16 AGEP most commonly affects adults, with a mean age of disease onset of 56 years.17
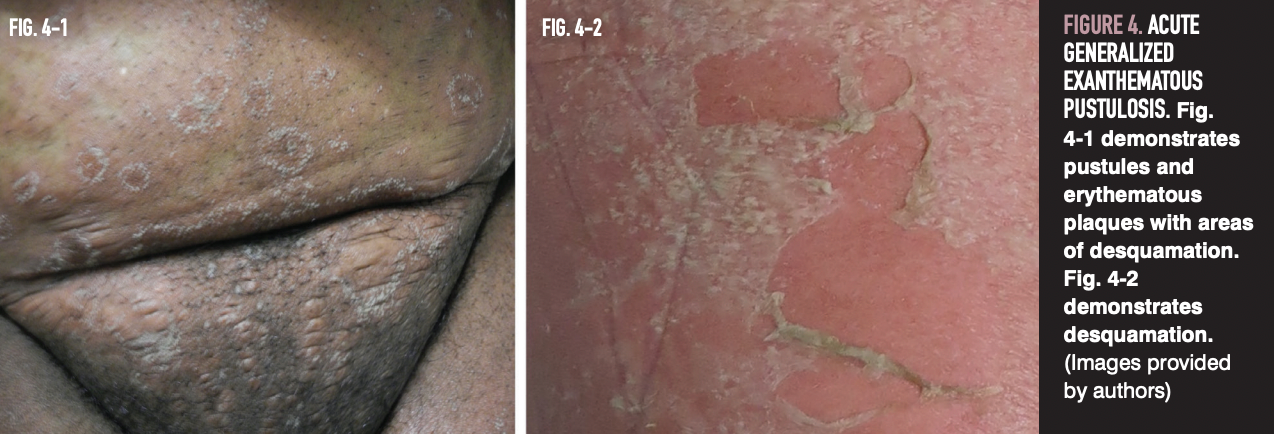
Skin involvement begins within a few hours to days after starting medication and is characterized by dozens to hundreds of 1-mm pustules on an erythematous base (Figure 4). A shorter interval between drug administration and AGEP onset is seen with antibiotics. Classically, involvement starts on the face or intertriginous areas and progresses to “lakes of pus” that are formed by coalescing pustules. Oral and genital mucositis, blisters, and erosions are less common features seen in AGEP.18,19 Associated systemic features are common, including fever, leukocytosis with a neutrophil count of greater than 7000, and eosinophilia. Approximately 20% of cases present with internal organ dysfunction, hypotension, and, rarely, shock.20,21
Diagnosis of AGEP is suspected when patients present with acute onset of febrile pustular eruption after starting antibiotics or other associated drugs and is supported by histopathologic characteristics on skin biopsy. Atypical manifestations, especially with systemic features, may present similarly to TEN.22 Patch testing can be useful in identifying the causative drug.23 Management involves withdrawal of the causative drug and topical corticosteroids for symptomatic treatment of pruritus. AGEP is self-limiting, and with withdrawal of the o ending agent, skin findings typically resolve within 1 to 2 weeks.24
Toxic erythema of chemotherapy
Toxic erythema of chemotherapy (TEC) is a general term that refers to the spectrum of nonallergic cutaneous eruptions caused by chemotherapeutics. Pathogenesis involves the excretion of cytotoxic compounds into eccrine sweat ducts, resulting in localized toxic effects.25-28 Common offenders include cytarabine, anthracyclines, 5- fluorouracil, capecitabine, taxanes, and methotrexate.26 TEC has also been associated with targeted therapies, including multikinase inhibitors.29
Most patients affected with TEC experience a palmoplantar dysesthesia that begins in the days to weeks post chemotherapy as tingling that progresses to burning.30 These neurological findings are accompanied by palmoplantar erythema and edema, most prominent on lateral aspects of fingers and distal fat pads.30,31
Of note, a subset of TEC involves intertriginous skin and is termed malignant intertrigo (Figure 5). Affected areas can include cervical folds, inframammary and inguinal folds, abdominal folds, antecubital fossae, axillae, perineum, buttocks, and thighs.25 Intertriginous involvement in TEC is frequently misdiagnosed, resulting in inappropriate management.25
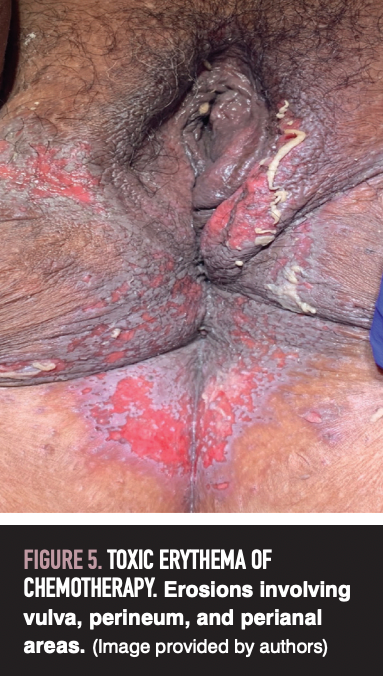
TEC is mostly a clinical diagnosis. If the diagnosis is unclear, biopsy may be required. Marked pain and tenderness is useful in differentiating malignant intertrigo from other intertriginous rashes.25 Symptom onset and the identified culprit drug may help differentiate malignant intertrigo from symmetrical drug-related intertriginous and flexural exanthema (SDRIFE), as SDRIFE is more commonly associated with antibiotics and presents on a more acute timeline, in the hours to days following administration of the offending drug.25,32 Management is supportive and includes cool compresses, analgesics, topical corticosteroids or antibiotics, and ointments for erosions.26 TEC and malignant intertrigo are self-limiting, with symptoms usually resolving within 1 to 2 weeks of treatment cessation.33 Severe cases may require systemic steroids to improve impact of quality of life and reduce the need to adjust or hold chemotherapy. Prophylactic measures include application of urea-based creams (for hyperkeratotic areas like the hands and feet), antiperspirants, and regional cooling.34-36
References
1. Arnold JD, Yoon S, Kirkorian AY. The national burden of inpatient dermatology in adults. J Am Acad Dermatol. 2019;80(2):425-432. doi:10.1016/j.jaad.2018.06.070
2. Comstock JR, Endo JO, Kornik RI. Adequacy of dermatology and ob-gyn graduate medical education for inflammatory vulvovaginal skin disease: a nationwide needs assessment survey. Int J Womens Dermatol. 2020;6(3):182-185. doi:10.1016/j.ijwd.2020.01.008
3. Hsu DY, Brieva J, Silverberg NB, Silverberg JI. Morbidity and mortality of Stevens-Johnson syndrome and toxic epidermal necrolysis in United States adults. J Invest Dermatol. 2016;136(7):1387-1397. doi:10.1016/j.jid.2016.03.023
4. Micheletti RG, Chiesa-Fuxench Z, Noe MH, et al. Stevens-Johnson syndrome/toxic epidermal necrolysis: a multicenter retrospective atudy of 377 adult patients from the United States. J Invest Dermatol. 2018;138(11):2315-2321. doi:10.1016/j.jid.2018.04.027
5. Niemeijer IC, van Praag MC, van Gemund N. Relevance and consequences of erythema multiforme, Stevens-Johnson syndrome and toxic epidermal necrolysis in gynecology. Arch Gynecol Obstet. 2009;280(5):851-854. doi:10.1007/s00404-009-1008-1
6. O’Brien KF, Bradley SE, Mitchell CM, Cardis MA, Mauskar MM, Pasieka HB. Vulvovaginal manifestations in Stevens-Johnson syndrome and toxic epidermal necrolysis: prevention and treatment. J Am Acad Dermatol. 2021;85(2):523-528. doi:10.1016/j.jaad.2019.08.031
7. Hoffman M, Chansky PB, Bashyam AR, et al. Long-term physical and psychological outcomes of Stevens-Johnson syndrome/toxic epidermal necrolysis. JAMA Dermatol. 2021;157(6):712-715. doi:10.1001/jamadermatol.2021.1136
8. Dodiuk-Gad RP, Olteanu C, Feinstein A, et al. Major psychological complications and decreased health-related quality of life among survivors of Stevens-Johnson syndrome and toxic epidermal necrolysis. Br J Dermatol. 2016;175(2):422-424. doi:10.1111/bjd.14799
9. Bernett CN, Fong M, Yadlapati S, Rosario-Collazo JA. Linear IGA Dermatosis. StatPearls; 2022.
10. Juratli HA, Sárdy M. Lineare IgA-Dermatose. Hautarzt. 2019;70(4):254-259. Lineare IgA-Dermatose. doi:10.1007/s00105-019-4377-9
11. Lammer J, Hein R, Roenneberg S, Biedermann T, Volz T. Drug-induced linear IgA bullous dermatosis: a case report and review of the literature. Acta Derm Venereol. 2019;99(6):508-515. doi:10.2340/00015555-3154
12. Fortuna G, Salas-Alanis JC, Guidetti E, Marinkovich MP. A critical reappraisal of the current data on drug-induced linear immunoglobulin A bullous dermatosis: a real and separate nosological entity? J Am Acad Dermatol. 2012;66(6):988-994. doi:10.1016/j.jaad.2011.09.018
13. Fortuna G, Marinkovich MP. Linear immunoglobulin A bullous dermatosis. Clin Dermatol. 2012;30(1):38-50. doi:10.1016/j.clindermatol.2011.03.008
14. Genovese G, Venegoni L, Fanoni D, Muratori S, Berti E, Marzano AV. Linear IgA bullous dermatosis in adults and children: a clinical and immunopathological study of 38 patients. Orphanet J Rare Dis. 2019;14(1):115. doi:10.1186/s13023-019-1089-2
15. Chen S, Mattei P, Fischer M, Gay JD, Milner SM, Price LA. Linear IgA bullous dermatosis. Eplasty. 2013;13:ic49.
16. Roujeau JC, Bioulac-Sage P, Bourseau C, et al. Acute generalized exanthematous pustulosis. Analysis of 63 cases. Arch Dermatol. 1991;127(9):1333-1338.
17. Sidoroff A, Dunant A, Viboud C, et al. Risk factors for acute generalized exanthematous pustulosis (AGEP)-results of a multinational case-control study (EuroSCAR). Br J Dermatol. 2007;157(5):989-996. doi:10.1111/j.1365-2133.2007.08156.x
18. Lee HY, Chou D, Pang SM, Thirumoorthy T. Acute generalized exanthematous pustulosis: analysis of cases managed in a tertiary hospital in Singapore. Int J Dermatol. 2010;49(5):507-512. doi:10.1111/j.1365-4632.2010.04313.x
19. Lin JH, Sheu HM, Lee JY. Acute generalized exanthematous pustulosis with erythema multiforme-like lesions. Eur J Dermatol. 2002;12(5):475-478.
20. Leclair MA, Maynard B, St-Pierre C. Acute generalized exanthematous pustulosis with severe organ dysfunction. CMAJ. 2009;181(6-7):393-396. doi:10.1503/cmaj.090137
21. Szatkowski J, Schwartz RA. Acute generalized exanthematous pustulosis (AGEP): a review and update. J Am Acad Dermatol. 2015;73(5):843-848. doi:10.1016/j.jaad.2015.07.017
22. Kostopoulos TC, Krishna SM, Brinster NK, Ortega-Loayza AG. Acute generalized exanthematous pustulosis: atypical presentations and outcomes. J Eur Acad Dermatol Venereol. 2015;29(2):209-214. doi:10.1111/jdv.12721
23. Mashiah J, Brenner S. A systemic reaction to patch testing for the evaluation of acute generalized exanthematous pustulosis. Arch Dermatol. 2003;139(9):1181-1183. doi:10.1001/archderm.139.9.1181
24. Alniemi DT, Wetter DA, Bridges AG, et al. Acute generalized exanthematous pustulosis: clinical characteristics, etiologic associations, treatments, and outcomes in a series of 28 patients at Mayo Clinic, 1996-2013. Int J Dermatol. 2017;56(4):405-414. doi:10.1111/ijd.13434
25. Smith SM, Milam PB, Fabbro SK, Gru AA, Kaffenberger BH. Malignant intertrigo: a subset of toxic erythema of chemotherapy requiring recognition. JAAD Case Rep. 2016;2(6):476-481. doi:10.1016/j.jdcr.2016.08.016
26. Bolognia JL, Cooper DL, Glusac EJ. Toxic erythema of chemotherapy: a useful clinical term. J Am Acad Dermatol. 2008;59(3):524-529. doi:10.1016/j.jaad.2008.05.018
27. Choi JN. Chemotherapy-induced iatrogenic injury of skin: new drugs and new concepts. Clin Dermatol. 2011;29(6):587-601. doi:10.1016/j.clindermatol.2011.08.032
28. Garcia-Navarro X, Puig L, Fernández-Figueras MT, Alomar A. Eccrine squamous syringometaplasia secondary to pegylated liposomal doxorubicin. Arch Dermatol. 2008;144(10):1402-1403. doi:10.1001/archderm.144.10.1402
29. McLellan B, Kerr H. Cutaneous toxicities of the multikinase inhibitors sorafenib and sunitinib. Dermatol Ther. 2011;24(4):396-400. doi:10.1111/j.1529-8019.2011.01435.x
30. Miller KK, Gorcey L, McLellan BN. Chemotherapy-induced hand-foot syndrome and nail changes: a review of clinical presentation, etiology, pathogenesis, and management. J Am Acad Dermatol. 2014;71(4):787-794. doi:10.1016/j.jaad.2014.03.019
31. Sibaud V, Dalenc F, Chevreau C, et al. HFS-14, a specific quality of life scale developed for patients suffering from hand-foot syndrome. Oncologist. 2011;16(10):1469-1478. doi:10.1634/theoncologist.2011-0033
32. Häusermann P, Harr T, Bircher AJ. Baboon syndrome resulting from systemic drugs: is there strife between SDRIFE and allergic contact dermatitis syndrome? Contact Dermatitis. 2004;51(5-6):297-310. doi:10.1111/j.0105-1873.2004.00445.x
33. Degen A, Alter M, Schenck F, et al. The hand-foot-syndrome associated with medical tumor therapy - classification and management. J Dtsch Dermatol Ges. 2010;8(9):652-661. doi:10.1111/j.1610-0387.2010.07449.x
34. Ren Z, Zhu K, Kang H, et al. Randomized controlled trial of the prophylactic effect of urea-based cream on sorafenib-associated hand-foot skin reactions in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2015;33(8):894-900. doi:10.1200/JCO.2013.52.9651
35. Templeton AJ, Ribi K, Surber C, et al. Prevention of palmar-plantar erythrodysesthesia with an antiperspirant in breast cancer patients treated with pegylated liposomal doxorubicin (SAKK 92/08). Breast. 2014;23(3):244-249. doi:10.1016/j.breast.2014.02.005
36. Scotté F, Banu E, Medioni J, et al. Matched case-control phase 2 study to evaluate the use of a frozen sock to prevent docetaxel-induced onycholysis and cutaneous toxicity of the foot. Cancer. 2008;112(7):1625-1631. doi:10.1002/cncr.23333

Newsletter
Get the latest clinical updates, case studies, and expert commentary in obstetric and gynecologic care. Sign up now to stay informed.
Current treatments for recurrent bacterial vaginosis leave many patients dissatisfied
February 28th 2025A new study presented at ISSWSH highlights patient dissatisfaction with current treatments for recurrent bacterial vaginosis, emphasizing the need for more effective therapies and improved provider communication.
Read More
