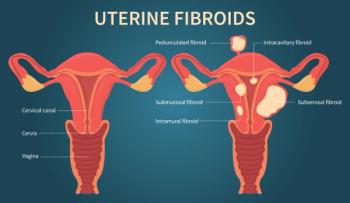
The privately held medical device firm Memic Innovative Surgery Ltd. announced in early March that the Food and Drug Administration (FDA) has granted De Novo marketing authorization for its Hominis, a robot-assisted surgical platform for use in single site, natural orifice laparoscopic-assisted transvaginal benign gynecologic procedures, including benign hysterectomy.