
Findings show that that feeding mode is by far the most defining factor for microbial composition," wrote the study authors.

Joshua Fitch is the senior editor for Contemporary OB/GYN. He joined the brand in January of 2026, after being the senior editor of our sister publication, Contemporary Pediatrics, since March of 2023. Fitch graduated from Youngstown State University in Youngstown, Ohio, in 2020 with a degree in telecommunications and journalism. He started his career as a news and sports videographer before becoming an on-air sports anchor at the NBC-affiliated news station in Youngstown. Fitch briefly worked as a national content writer for a Chicago-based national television station before joining the Contemporary Pediatrics team. He can be reached at: jfitch@mjhlifesciences.com.

Findings show that that feeding mode is by far the most defining factor for microbial composition," wrote the study authors.

A JAMA Pediatrics study found 37% of adolescents with gender dysphoria began hormone therapy within 2 years, highlighting barriers and inequities in care.

The third and final episode in our series looks at what is in the pipeline as well as a discussion around FDA guidance.

A CDC investigation is underway after a Listeria outbreak, connected to meats sliced at delis, resulted in multiple hospitalizations and fatalities, urging high-risk groups to take precautions.

The second episode in our series looks to address clinical management in a time when antimicrobial resistance appears to be growing.
Explore the latest findings revealing vaccination rates among children aged 9 to 17 years, uncovering demographic disparities and highlighting the crucial role of vaccination in combating human papillomavirus infections and related health risks.

Investigating the impact of prenatal vitamin C supplementation on lung function and wheeze occurrence in offspring of pregnant smokers, revealing insights into mitigating respiratory risks.

Discover how maternal tobacco usage during pregnancy affects memory and language skills in children aged 9 to 12 years, as revealed by a comprehensive cohort study.

Samantha Olson, MPH, highlights how maternal vaccination protects infants from influenza.
A recent study found that in utero exposure to maternal COVID-19 vaccination poses no increased risk for neurodevelopmental impairment in infants up to 18 months of age, addressing concerns about the impact of vaccine exposure on unborn children.
In the third installment of RSV Roundtable, our panel discusses the impact nirsevimab has had this RSV season.
In the second installment of RSV Roundtable, our panel explains how they are educating patients and parents when it comes to RSV, vaccines and preventive measures, and limited treatment availability.

A 755% increase in reported cases since 2012 highlights the need for timely testing and treatment to prevent maternal and infant health risks.

The combination vaccine candidates demonstrated a safety profile consistent with Pfizer’s COVID-19 vaccine. A phase 3 trial is anticipated to commence in the coming months, according to a press release from Pfizer.

Mary Jane Minkin, MD, FACOG, MSCP, discusses key takeaways from The Menopause Society 2023 Annual Meeting, held in Philadelphia, Pennsylvania from September 27-30.

Through a randomized, multicenter, open-label, controlled study, investigators determined there were no suggestions of important differences for very preterm infant body composition when comparing an exclusive human milk diet to a diet containing cow milk products.

In collaboration with Contemporary Pediatrics, Contemporary OB/GYN, and Contagion, Tina Tan, MD, FAAP, FIDSA, FPIDS, discusses the benefits of having new tools to fight RSV in infants, potential vaccine hesitancy, and RSV trends currently being observed.

Ahead of the first fall and winter virus seasons in which vaccines are available for COVID-19, influenza, and respiratory syncytial virus (RSV), the Centers for Disease Control and Prevention is recommending Pfizer’s maternal vaccine to protect newborns from severe RSV illness.

Francheska M. Merced-Nieves, PhD, Assistant professor, Departments of Pediatrics and the Institute for Exposomic Research of Environmental Medicine & Public Health, Icahn School of Medicine at Mount Sinai, explains the associations prenatal exposure to a metal mixture and the potential negative effects for the infant.

A targeted, collaborative approach to reduce length of stay (LOS) after cesarean or vaginal delivery resulted in a significant and sustained decrease in mean postpartum LOS without compromising patient safety, according to a poster featured at the 2023 Society of OB/GYN Hospitalists Annual Clinical Meeting, held in Chicago, Illinois.

Matthew Zerden, MD, provides various tips and resources for ob-gyn hospitalists wanting to implement postpartum LARCs in their institution, according to his presentation at the 2023 Society of OB/GYN Hospitalists Annual Clinical Meeting in Chicago, Illinois.

John Stanley, MD, details how providers can create an OB hospitalist program , according to his presentation at the 2023 Society of OB/GYN Hospitalists Annual Clinical Meeting in Chicago, Illinois.
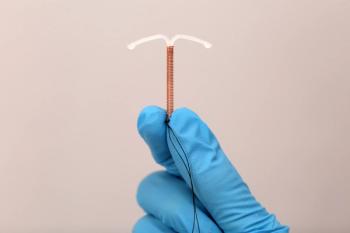
The IPP LARC program “allowed for the provision of LARCs for women who had limited to no funds or access, especially in the postpartum period,” according to study authors of a poster presented at the 2023 Society of OB/GYN Hospitalists Annual Clinical Meeting in Chicago, Illinois.

Marci Bowers, MD, shares the biggest takeaways from her presentation on transgender reproductive care, presented at the 2023 Society of OB/GYN Hospitalists Annual Clinical Meeting in Chicago, Illinois.

Sean Esplin, MD, maternal-fetal medicine physician, senior medical director for women’s health, professor, Department of OB/GYN, University of Utah, discusses his presentation on advanced fetal heart rate monitoring interpretation at the 2023 Society of OB/GYN Hospitalists Annual Clinical Meeting in Chicago, Illinois.

Jermaine Gray, MD, provides some essential tips for various cases of vulvovaginal conditions that present in the emergency department, which was recently presented at the 2023 Society of OB/GYN Hospitalists Annual Clinical Meeting.

Results from a case study analysis submitted during the 2023 Society of OB/GYN Hospitalists Annual Clinical Meeting in Chicago, Illinois, highlight the potential of ketamine as a potential alternative to traditional antidepressants for postpartum depression (PPD) treatment.

Angela Dempsey, MD, MPH, gives her insights on how to advocate for your patients in post-Roe America, which was presented during her session at the recent 2023 Society of OB/GYN Hospitalists Annual Clinical Meeting in Chicago, Illinois.

Approved for use at 32 weeks through 36 weeks gestation, Pfizer’s maternal respiratory syncytial vaccine (Abrysvo), is delivered through a single dose injection to the muscle, and is the first vaccine for use in pregnant individuals to prevent lower respiratory tract disease (LRTD) and severe LRTD because of RSV in infants (birth to 6 months).

According to Pfizer, the investigational vaccine to protect against Group B Streptococcus (GBS) generated maternal antibody responses against 6 capsular polysaccharide serotypes and efficiently transferred antibodies to the infants. The announcement of this phase 2 data comes in July, which is International Group B Streptococcus Month.