
A panel discussion at the 2021 American College of Obstetrics and Gynecology’s (ACOG) Annual and Scientific Meeting, being held virtually April 30-May 2, offers insight into how the COVID-19 pandemic has changed telehealth in ob/gyn.

A panel discussion at the 2021 American College of Obstetrics and Gynecology’s (ACOG) Annual and Scientific Meeting, being held virtually April 30-May 2, offers insight into how the COVID-19 pandemic has changed telehealth in ob/gyn.

Round two of the program will provide $249 million to support physicians by providing reimbursement of services necessary for telehealth.
The CDC and FDA will further investigate the reports of blood clots following administration of the vaccine.

Treating COVID-19 in pregnant patients brings extra challenges, risks.
The pandemic has forced us to get more creative in managing patients’ needs, especially when it comes to contraception

The National Institutes of Health (NIH) announced that it will offer grant extensions to eligible grantees affected by COVID-19.

The Centers for Disease Control and Prevention (CDC) has issued interim guidance for masking, social distancing, hand hygiene, and other infection control measures for people who are fully vaccinated for coronavirus 2019 disease (COVID-19).

In a briefing this morning, the Infectious Diseases Society of America (IDSA) stressed the continued importance of coronavirus 2019 disease (COVID-19) control measures that have been there from the start.

President Biden promised that the federal government will have enough vaccine supply to vaccinate approximately 300 million Americans by the end of May.

Diverse Voices: COVID-19 and the Health of Women, a virtual speaker series run by the NIH Office of Research on Women’s Health (ORWH), presented compiled research on sex and gender disparities during the COVID-19 pandemic. In addition to identifying issues of concern, the webinar offered actions to improve women’s health despite sex and gender disadvantages.
The World Health Organization (WHO) had previously issued guidance recommending that pregnant women should not receive the Moderna Inc. vaccine unless the individual is at high risk of exposure or having a severe case.
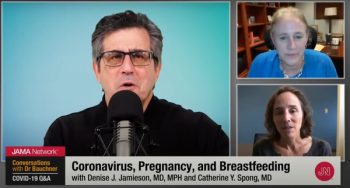
Contemporary OB/GYN’s Editor in Chief, Catherine Y. Spong, MD, recently joined Howard Bauchner, MD, Editor in Chief of the Journal of the American Medical Association (JAMA) and Denise J. Jamieson, MD, MPH for a Q&A session to discuss COVID vaccination in pregnant and breastfeeding women.

This article is on based on information presented at the Society for Maternal-Fetal Medicine’s 2021 Virtual Annual Meeting, which will be held from Jan. 25 to Jan. 30.
The World Health Organization (WHO) had previously issued guidance recommending that pregnant women should not receive the Moderna Inc. vaccine unless the individual is at high risk of exposure or having a severe case.

On this episode of Pap Talk, Emily Adhikari, MD answers frequently asked questions about the COVID-19 vaccine.
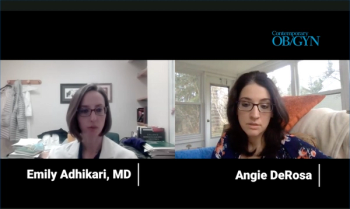
In this video, Senior Editor Angie DeRosa interviews Emily Adhikari, MD, on questions or concerns she and colleagues are hearing from patients surrounding the COVID-19 vaccine.

The pandemic brought telehealth into the mainstream, but will government and private payers make the right moves to make it a standard part of medicine?
This FAQ is a printable reference for ob/gyns. It features information on the COVID-19 vaccines, as well as frequently asked questions and answers for ob/gyns to use when counseling patients.
The recommendations address the role of physicians as vaccinators.

Data on COVID-19 during pregnancy, as reported by the CDC, in collaboration with state, local, and territorial health departments and external partners.
In this video interview, Senior Editor Angie DeRosa talks with Linda Fan, MD, about the rise of telehealth as the pandemic enters the second stage.
U.S. FDA staff have recommended monitoring individuals who get the Pfizer or Moderna vaccine shots for possible cases of Bell’s palsy.


COVID-19 exacerbated the global need for reliable healthcare information, but the unparalleled urgency and shrinking timeline made things difficult for providers.

Data on COVID-19 during pregnancy, as reported by the CDC, in collaboration with state, local, and territorial health departments and external partners.