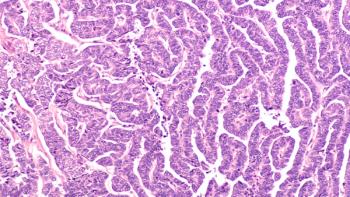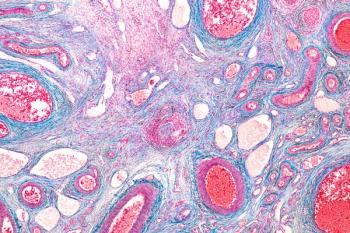
The combination of lenvatinib plus pembrolizumab induced durable responses with a manageable safety profile in adults with previously treated, advanced endometrial cancer, according to a long-term follow-up analysis of a phase 1b/2 study (NCT02501096).