
Clinicians conducting breast examinations who don’t apply enough force during palpation risk missing deeper lesions.

Clinicians conducting breast examinations who don’t apply enough force during palpation risk missing deeper lesions.

Among BRCA mutation carriers, risk-reducing bilateral salpingo-oophorectomy (RRSO) results in an 80% reduction in risk of ovarian cancer and a 50% reduction in risk of breast cancer if the surgery is performed before the onset of menopause.

Genetic testing for ovarian or endometrial cancer isn't recommended for everyone. Find out here which patients should be referred for testing.

A review of the latest research on the persistence of vasomotor symptoms, the link between hormone therapy and ovarian cancer risk, and the prevalence of substance use in pregnant adolescents.
Ilene Gewirtz, MD, discusses her comprehensive approach to well visits and cancer screens and says education, if not expanded services, is key.

Even brief use of hormone replacement therapy in menopause can increase risk of the 2 most common forms of ovarian cancer, a meta-analysis finds.
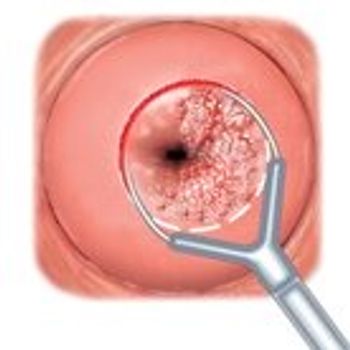
Treatments to remove precancerous cervical lesions don't seem to affect a woman's ability to become pregnant, new research found.
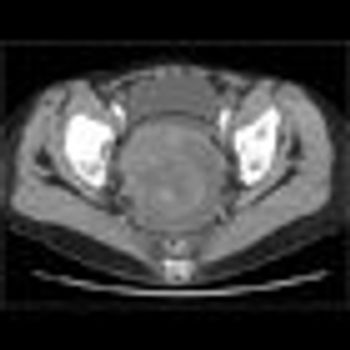
Could the approval of bevacizumab for advanced cervical cancer and platinum-resistant ovarian cancer last year lead to patient-specific therapies?

Vaginal estrogens are effective options for managing bothersome symptoms related to genitourinary syndrome of menopause in postmenopausal women.
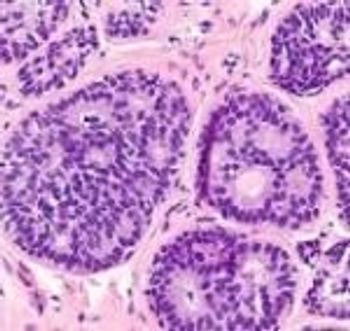
New evidence reveals that risk prediction for breast cancer is imperative in women with atypical hyperplasia of breast tissue.

According to a new study in Gynecologic Oncology, women who have polycystic ovary syndrome (PCOS) may be at greater risk of developing some types of cancer.

An ADP-ribose polymerase (PARP) inhibitor has received accelerated approval from the US Food and Drug Administration (FDA) for treatment of women with heavily pretreated ovarian cancer associated with defective BRCA genes.

Women at risk of ovarian cancer and who are undergoing hysterectomy should be counseled about the possible benefits of salpingectomy, according to a new committee opinion from the American College of Obstetricians and Gynecologists.

The FDA has approved olaparib, to be marketed under the name Lynparza, for the treatment of advanced ovarian cancer related to defective BRCA genes.

Addressing the emotional component of a patient's diagnosis isn't often feasible, but these tools can help patients with this important aspect of healing.

On December 10, 2014, the US Food and Drug Administration (FDA) approved Gardasil 9 (Human Papillomavirus [HPV] 9-valent Vaccine, Recombinant) for the prevention of certain diseases caused by 9 types of HPV.

A new (theoretical) model using multiple data points, including genetic testing for BRCA genes, could identify women at greatest risk for breast cancer.

Studies of the impact of diet on risk of ovarian cancer are limited and their results unclear but a new report based on data from the Nurses’ Health Study (NHS) suggests a possible association between high intake of flavonoids and black tea and lower risk of the disease.

Voters in North Dakota sent a clear message that health care decisions, from pregnancy to end-of-life care, must involve only patients and their physicians.
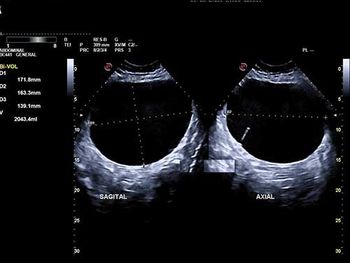
Challenge your diagnostic skills: What's causing this woman's pelvic discomfort?

A consequence of Measure 1, which may or may not be a "personhood" amendment, is that IVF and other infertility services in North Dakota will end.

cfDNA gives insight into the pathogenesis of serious disease and early information about benign conditions.

Two experts discuss whether a DNA test can replace the Pap.
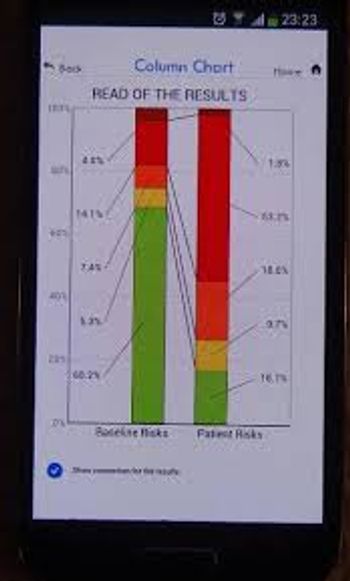
A new app, called ADNEX, helps distinguish between benign and malignant ovarian tumors, potentially improving triage and management decisions.

Breast cancer awareness campaigns are great, but they should be expanded to provide more clinician education on health disparities and to target women most at risk.