
Gastric Lap Band Surgery For Weight Loss

Today we are starting a new series on OBGYN.net about treatments for non-ob/gyn conditions that some women will choose to have in their lifetime. Roberta Speyer, owner and publisher of OBGYN.net, traveled to Frankfurt, Germany in December for a treatment for obesity that is not FDA-approved in the United States. This surgery as well as other topics will be highlighted in the months to come.

OBGYN.net is planning a new section for our web site. We are dedicating a section to both the wellness of women and to general topics of special interest to women. We are starting to develop these new topics here on the Women's Home Page in preparation for the new section, which should be online this summer.

OBGYN.net Conference CoverageFrom Radiological Society of North America (RSNA)Chicago, Illinois, November 2000


Test your ob/gyn knowledge in our DailyDx.

Uterine Fibroids are classified by their location (see figure), which effects the symptoms they may cause and how they can be treated. Fibroids that are inside the cavity of the uterus (Submucous myomas) will often cause bleeding between periods and often cause severe cramping. Fortunately, these fibroids can usually be easily removed by a method called “hysteroscopic resection,” which can be done through the cervix without the need for an incision.

When chlamydia or gonorrhea is diagnosed in female patients, obstetricians and gynecologists should also prescribe antibiotics for the male partners. This recommendation comes from The American College of Obstetricians and Gynecologists in efforts to reduce the high reinfection rates associated with these sexually transmitted infections (STIs).

Botox (onabotulinumtoxinA) injection has been approved by the FDA to treat urinary incontinence caused be overactive bladder related to conditions such as spinal cord injury and multiple sclerosis. In persons with certain neurological conditions, uninhibited bladder contractions can make storing urine difficult. This condition traditionally has been managed with medication to relax the bladder and use of a catheter to empty the bladder.

Prophylactic human papillomavirus vaccines have been shown to be effective in reducing the disease burden of cervical cancer, but the three dose regimen can be expensive and difficult to complete. With that in mind, Dr Aimée R. Kreimer, investigator in the division of cancer epidemiology and genetics at the National Cancer Institute, the National Institute of Health, and colleagues sought to determine if less than three doses of the vaccine would be effective. The results are published in the Journal of the National Cancer Institute.

Laparoscopy is a minimally invasive surgical technique in which abdominal surgery is performed through tiny "keyhole" incisions on the abdomen.

To review utilisation of elective embryo cryopreservation in the expectant management of patients at risk for developing ovarian hyperstimulation syndrome (OHSS), and report on reproductive outcome following transfer of thawed embryos.

Dysplastic Ichthyosis Uteri-like Changes of the Entire Endometrium Associated with a Squamous Cell Carcinoma of the Uterine Cervix

The first vaccine to prevent human papillomavirus (HPV) and cervical cancer has been licensed, and in future, vaccination may be routinely offered to 10–14 year old girls. HPV is a sexually transmitted virus and some parents may refuse consent for vaccination.
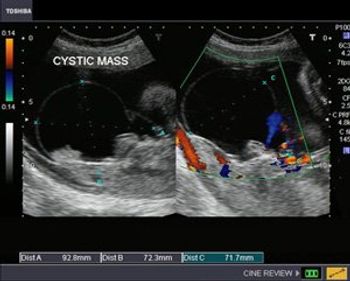
This case study shows a 26 week gestation with a cystic mass close to the sacrum.

Download the .PPT version of this presentation for off line viewing (1.92 MB)Return to Power Point Presentations Indexsubmit your own material for publication via email with your files attached

A recent study on sleep disturbances in menopause shows a direct connection to the use of hormone therapy. Specifically, when women stop taking hormone therapy they experienced greater periods of poor sleep, waking up during the night, difficulty falling asleep, waking up to early and not feeling rested.

According to results from a new study, a popular herbal remedy may come to the rescue of women suffering from postmenopausal sleep disturbances. About half of postmenopausal women experience some degree of sleep disturbance, which in turn impacts their quality of life.

Cessation of hormone therapy in menopausal women may result in sleep disturbances, according to a new study in Menopause. Since the hormone therapy has been associated with the alleviation of sleep problems in women experiencing menopausal symptoms, Dr Sarah E Tom, Interdisciplinary Women’s Health Research (IWHR) Scholar at the University of Texas Medical School, and colleagues sought to determine the resulting sleep effects during cessation of hormone therapy.

Since their introduction, cervical cytology screening programs have caused a substantial reduction in cervical cancer rates. However, the low sensitivity of one screen and resulting need for repeated screens during a lifetime to achieve programmatic sensitivity has deemed it inefficient. With that in mind, Dr Philip E Castle, of the American Society for Clinical Pathology in Washington, D.C., and colleagues compared the performance of the cobas human papillomavirus (HPV) test versus liquid-based cytology.

Physicians refer many women at average risk of ovarian cancer for genetic counseling or testing for the BRCA 1 and 2 genes while neglecting to refer many high-risk women, researchers reported in the July 25 online edition of Cancer.

Significance of the umbilical cord: the umbilical cord can be aptly termed the life line for the fetus during its intrauterine life but can often be the cause for its misery in case of cord pathology.


ACOG has recently released “Thromboembolism in Pregnancy” in the September 2011 issue of 'Obstetrics & Gynecology' to guide clinicians in the prevention, management and treatment of blood clots during pregnancy.

Accurate diagnosis of uterine fibroids is essential in deciding if treatment is necessary, and planning appropriate treatment.n While a physical exam may suggest fibroids, other conditions such as ovarian cysts or adenomyosis may be mistaken for fibroids. For this reason, I routinely do an ultrasound examination at the time of the first visit when a woman has symptoms of abnormal bleeding or cramping, or if I feel an abnormality on examination.

OBGYN.net Conference CoverageFrom American Association of Reproductive Medicine55th Annual Meeting of ASRM held conjointly with CFAS- Toronto, Ontario, Canada - September, 1999

Maternal use of opioid analgesics just prior to, or during the first trimester of, pregnancy increases the risk for certain congenital heart defects among offspring.

Although overall impact on bone is small and depends largely on age and dose, data suggest that by about 2 years of use, teens who use oral contraceptives show about 1% less gain in bone mineral density than teens who do not use the pills.

The overall birth count and fertility rate in the US declined in 2010.